Atoms are indivisible particles as proposed by Dalton's Atomic theory, is it true? Absolutely not.
Comb dry hair. Does the comb then
attract small pieces of paper? Rub a glass rod with a silk cloth and
bring the rod near an inflated balloon.
Observe what happens.
If atoms are indivisible, then why they get charged.
(pichle episode me aapne Dalton ji ki atomic theory padhi, jisme unhone ye kaha tha ki atoms can not be further divisible. Agar atoms divisible nhi hai to har element ke atom alag kyo hote hai. Dry hair me comb karne se comb charge kyo hoti hai. Ye sab ko vaise samjhane ki liye pehle page pr bataya hua hai. Agar atom indivisible hota to ye chapter kyo aata. Simple si baat hai agar hamare syllabus me hai to atom indivisible to hoga nahi. To chalo fir, aaj padhte hai structure of atom. Ye notes vaise NCERT based hai aur sufficient hai. Baki agar koi jaruri point hoga to vo bhi tumhe bata denge jo book me nahi hai. Vaise to book me ye chapter discovery of electron and proton se start hai par pehle mai aapko Atomic models bataunga kyuki uske bina aapko starting ka part samajh nhi aayega. )
THOMSON's MODEL OF ATOM
J.J Thomson proposed his atomic model in 1898. His model was also known as plum pudding model or watermelon model. In 1897, he discovered electrons through his cathode ray tube experiment.The electrons, in a sphere of positive charge,
were like currants (dry fruits) in a spherical
Christmas pudding.
Or the electrons are embedded just like the seeds of watermelon. The seeds are negatively charged and the red part of watermelon is positively charged. To explain the stability of atom, he said that the magnitude of positive charged surface is equal and opposite to negatively charged electrons
Thomson proposed that:
(i) An atom consists of a positively
charged sphere and the electrons are
embedded in it.
(ii) The negative and positive charges are
equal in magnitude. So, the atom as a
whole is electrically neutral.
(Thomson ne 1897 me electrons ko discover kiya tha ek experiment ka use karke. Us experiment ka naam cathode ray experiment tha. Bas naam yaad rakhna kyonki is experiment ke bare me tumhare syllabus mai nahi hai. Lekin jo senior classes mai medical ya non-medical lenge unhe ye experiment bhi padhna padega. Fir unhone 1898 mai apna atomic model present kara jo ki world ka sabse pehla atomic model tha. Ise samajhane ke liye tumhe watermelon ya tarbooz ka pata hona chahiye. Ab jinhe tarbooz ka bhi nahi pata vo pehle tarbooz kha kar aao. Haan to unhone ye kaha tha ki atom ek tarbooz ki tarah hai jisme electrons ya negatively charged part positive part me embedded yani ki dhasa hua hai. Yani ki atom ke andar positive or negative dono tarah ke charge hote hai. To Atom ki stability explain karne ke liye inhone keh diya ki in dono ka magnitude same hai. Yani ki jitna electrons negative hai utna hi positive part positive hai. Ek baat yaad rakhna ki abhi hum protons ki baat nhi kar rahe hai kyoki abhi tak protons discover nahi hue hai. Thomson ke model nai atom ki electronic stability ko to explain kar diya par dusre scientists ke models ke result ko ye model explain nhi kar paya. )
RUTHERFORD' s MODEL OF ATOM
Ernest Rutherford proposed his atomic model in 1911. He performed an alpha particle scattering experiment in which he made several observations and based on them, he made several predictions on how electrons are arranged inside an atom.
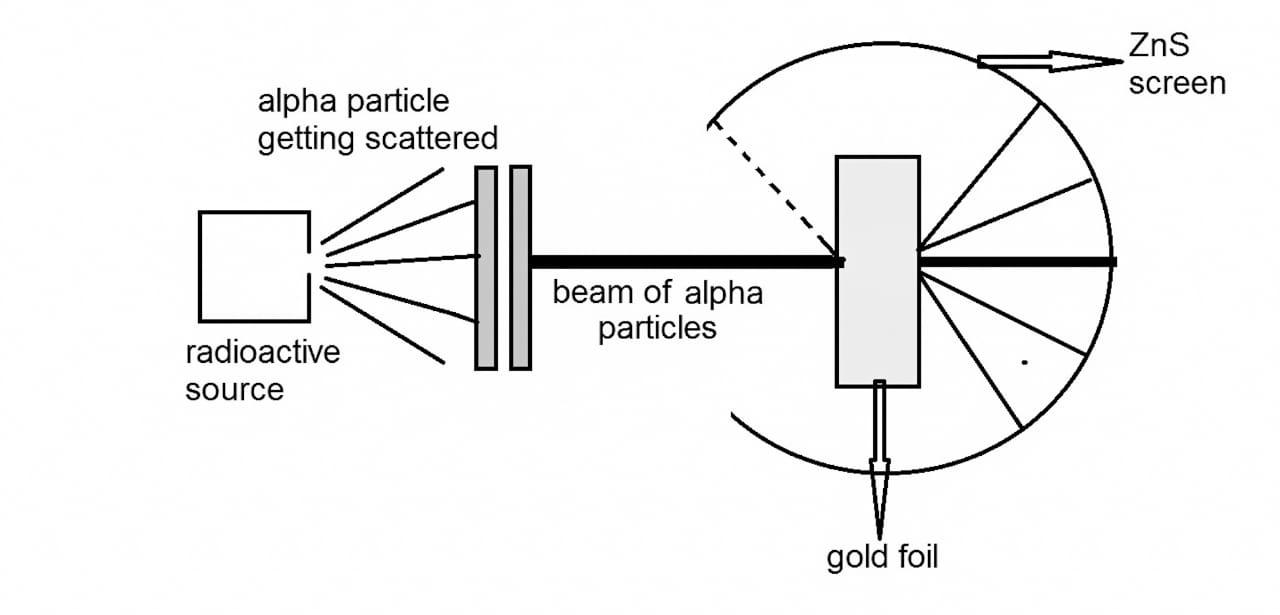
ALPHA PARTICLES SCATTERING EXPERIMENT
To understand this experiment you need to know about alpha particle, they are just Helium nuclei.
In this experiment, fast moving alpha
(α)-particles were made to fall on a thin
gold foil. He used gold foil because gold is the most malleable metal. The thickness of foil is about 1000 atoms. Alpha particles are positively charged and heavy particles. As charges of same polarity repel each other, so if the alpha particles are repelled then there must be a positive charge inside the atom. He use screen of Zinc Sulfide which glows whenever alpha particle is fallen on it. The source of alpha particle is placed inside a lead box because it helps to concentrate beam on foil.
(1911 me Rutherford ji ne bhi apna atomic model present kiya. Ye atomic model jis experiment pr based tha use alpha particle scattering experiment kehte hai or ye experiment tumhare syllabus me bhi hai. Is syllabus me se several questions puche jate hai. Jaise ki gold foil hi kyu use hui. Is experiment ka setup upar wali image me hai. Jisme alpha particle ka source Lead box ke ander rakha hua hai jisse alpha particle ki beam gold foil pr concentrate ho ske. Gold foil pr beam padne ke baad vo beam jaise hi screen pr padegi vaise hi vo glow karegi taki deflection notice ho ske. Aur aplha particle ek radioactive beam hai. Helium ke atom mai se agar electron nikal do to alpha paricle banta hai. Ab electron ke nikal jane ke baad sirf positive charge hj reh jata hai aur isliye alpha particles positively charged hote hai. )
The experiment gave the following results:
(i) Most of the fast moving α-particles
passed straight through the gold foil.
(ii) Some of the α-particles were deflected
by the foil by small angles.
(iii) One out of every 12000
particles appeared to rebound.
(Jyada tar aplha particles gold foils mai se directly pass ho gaye. Kuch alpha particles kisi angle pr deflect hue aur 12000 me se ek beam bounce back yani ki jis path se gayi usi path se retrace ho gayi. Ab ye baat to normal si hai ki in result ke based pr hi rutherford ji ne observation conclude kiye the.)
Rutherford
concluded from the α-particle scattering
experiment that–
(i) Most of the space inside the atom is
empty because most of the α-particles
passed through the gold foil without
getting deflected.
(ii) Very few particles were deflected from
their path, indicating that the positive
charge of the atom occupies very little
space.
(iii) A very small fraction of α-particles
were deflected by 1800,indicating that
all the positive charge and mass of the
gold atom were concentrated in a very
small volume within the atom
(To dekho result me jyada tr aplha particles bina kisi hindrance ka paas ho gaye the isi liye ye conclusion kiya gaya ki atom ke andar ka jyada tar space empty hota hai. Bahut kam aplha particles ek angle pr deflect hue, aur ye maine pehle bhi bataya hai ki aplha particles positively charged hote hai aur unke deflect hone ka matlab hai ki atom ke andar positive charge hai aur kyoki ye number bahut kam tha to isse ye conclude kiya gaya ki jo positive part hota hai vo kafi kam space mai hai. )
Rutherford also calculated the size of nucleus which 10^5 time less than atom. Now to understand this difference, let me tell you that if the size of nucleus has the size of football, then the atom have a radius of 5km.
(Rutherford ne is experiment ki help se nucleus aur atom ka size ka difference bhi pata laga liya tha jo ki upar likha hai )
FEATURES OF RUTHERFORD MODEL
(i) There is a positively charged centre in
an atom called the nucleus. Nearly all
the mass of an atom resides in the
nucleus.
(ii) The electrons revolve around the
nucleus in circular paths.
(iii) The size of the nucleus is very small
as compared to the size of the atom.
(Haan ye bilkul ncert ki copy hai kyounki features me aur kya batau. Bas itna samajh lo ki jo atom ke andar ka positive part hai vo centre me hai aur atom ka sara mass usi centre yani ki positive part me concentrated hai. Agar atom ka size football stadium jitna hai to nucleus ka size ek matar ke dane jitna hoga. Vaise to ye sari chize microscopic level pr hai jinhe padhne ke liye quantum physics padhni padegi pr tum ye dekho ki hum sochte hai ki welding ke bad bich me space na bache aur atom me mostly space empty hota hai. Inhone ek baat aur kahi thi ki electrons nucleus ke around revolve yani ki chakkar lagate hai vo bhi circular path me. Ab yaha se tumhe ye clear ho gaya hoga ki kyo ye model thomson model ka drawback hai. Haan aur ye teesra vala point to mai bata hi chuka hu ki atom ke comparison me nucleus bahut chota hota hai)
DRAWBACKS OF RUTHERFORD MODEL
1 This model failed to explain the stability of atoms.
2. According to the model, electrons revolve around the positively charged nucleus. It's not possible for the long run as we know atoms are stable while any particle in a circular orbit would undergo acceleration. During acceleration charged particles would radiate energy. Revolving electrons will lose energy and finally fall into the nucleus.
( Ye model atoms ki stability ko explain nhi kr paya tha tha. Ab iska reason ye hai ki circular motion me koi bhi object apni speed badalta hai aur iski wajah jo uski energy discharge hogi jisse vo nucleus me gir jayega kuch is tarah se)
If you want part 2 then comment me




Comments
Post a Comment